Blue has always been one of the most popular colours in food and beverage. It is often used to convey a sense of naturalness, healthfulness, and refreshment. Blue is also a very versatile colour, as it can be used in a wide range of products.
There has been a lot of controversy over the years with Brilliant Blue, which is an artificial colour, due to its link with hyperactivity in children.
In this blog post, we talk about what can be used as a natural alternative and it’s stability towards certain environments.
Spirulina
Spirulina blue is derived from Arthrospira platensis, a type of Blue Green algae. With very few natural blue shades, Spirulina is a widely used option for achieving Clean-Label blue shades.

The primary pigment responsible for the dazzling blue colour of Spirulina is known as Phycocyanin, which has limited heat and acid stability. This means that your products must be carefully processed to avoid damaging the pigment. Below, we’ve shown an example of what happens to the shade of spirulina when the pH and temperature have been altered slightly.

Typically, spirulina isn’t bake stable, however with some tricks from our application kitchen, it made a rare appearance in our rainbow bread! Get in touch to find out how 😉

Stability Testing
The technical team at Plant-Ex are dedicated to improving stability and shelf-life of our products. Focusing on this, they tested the heat stability, light stability and pH stability of NC0010 spirulina to show you how the pigment is altered depending on the environment.
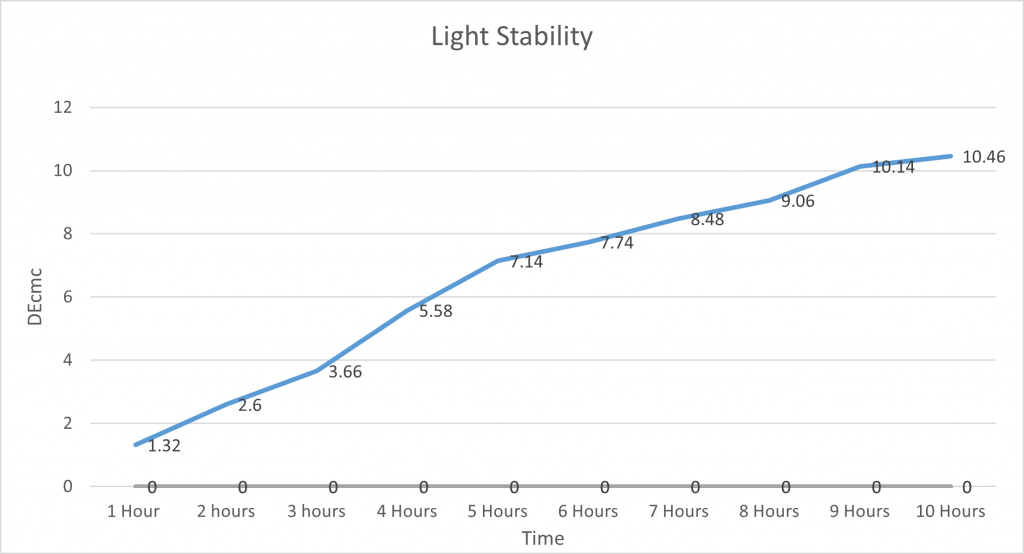
Light Stability:
In order to test the light stability of spirulina, the Colour Lab weighed 100g of Spirulina Solution into a beaker and then decanted it into a plastic bottle. This was labelled and placed into a dark cupboard as the control. They then took another 100g of spirulina solution, placed it into a separate plastic bottle and labelled it, and put it into direct sunlight. The Delta E was recorded using the x-rite every hour, and a photo was taken every 5 hours to show the difference in colour.
This graph shows that spirulina is quite stable against UV rays. As we left it outside for 10 hours and there was not a huge increase in DEcmc. It was gradual, unlike Heat and pH which more dramatically changed the DEcmc. Proving that spirulina is very unaffected by light and this means it does not need to be taken into too much consideration when storing it and using it in products/applications.
The below photos compare the control sample of NC0010 spirulina in solution, which was kept in a dark cupboard compared to the sample that was kept in the sunlight after 5 hours, and then 10 hours. As you can see, after 10 hours the colour has only slightly faded.


It is important to note this is in direct sunlight and is much stronger than the bulbs used in warehouses and factories, so it should be able to last much longer as even in high-intensity light it does not degrade quickly and is quite stable.
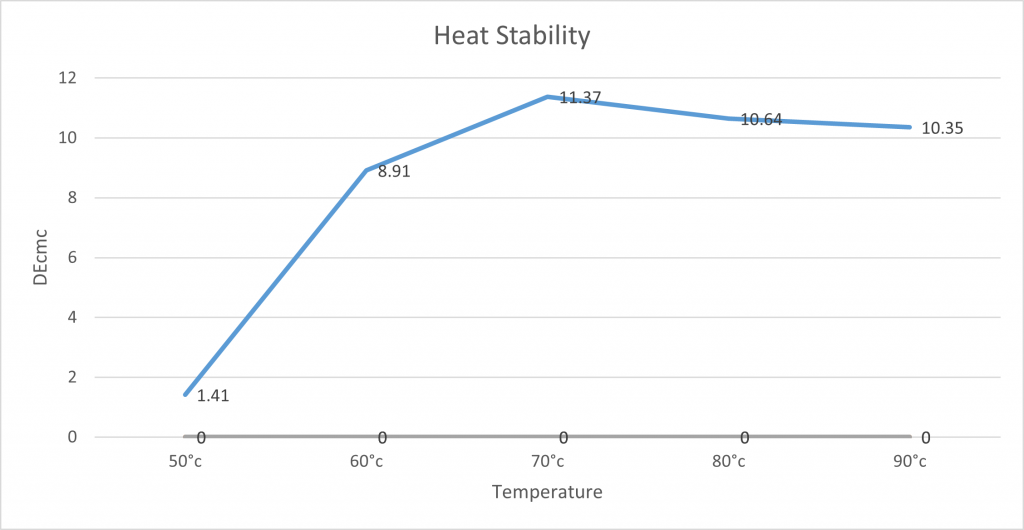
Heat Stability:
The lab tested the heat stability of spirulina by measuring 100g of spirulina solution and placing them onto hot plates to the desired temperature. Once it had reached this temperature, it was left for 10 minutes to see how the pigment was affected by the temperature over a certain amount of time. After 10 minutes, the beaker was removed from the hot plates and placed into an ice bath to control any further pigment and precipitate change. The samples were photographed and measured on the X-rite for comparison.
Whilst spirulina has good light stability, it is not stable to heat. The below graph demonstrates that when NC0010 spirulina is heated above 50°C, the colour will degrade and fade. This needs to be considered when being used in an application.
Below, you will see the photographs taken by our Colour Specialists. The beaker furthest to the left is the control, then you’ll see the beaker heated to 50 degrees Celsius, then 60 degrees Celsius and so on.

It is important to note that heavy amounts of precipitation were observable after 70°C, which is why we believe the DEcmc is lower due to the concentration of pigment. The graph shows there is a large difference in DEcmc between 50°C and 60°C. However from 60°C onwards it is more stable not affecting the DEcmc so drastically. However, it is a lot different to the control solution and discolouration is observable. Showing that beyond 50°c spirulina is not as stable.
pH stability:
The pH stability of spirulina was tested by measuring 100g of spirulina solution into a beaker, and the pH was measured using a pH probe. Next, the pH was lowered using citric acid with the results of this being recorded using our X-rite and a photo was taken. This was repeated at various pH levels.
The photo below shows the standard control sample of NC0010 in solution with its natural pH of neutral pH7. It demonstrates the observable change in colour when it reached pH 3, with it turning more of a grey with heavy amounts of pigment precipitate.

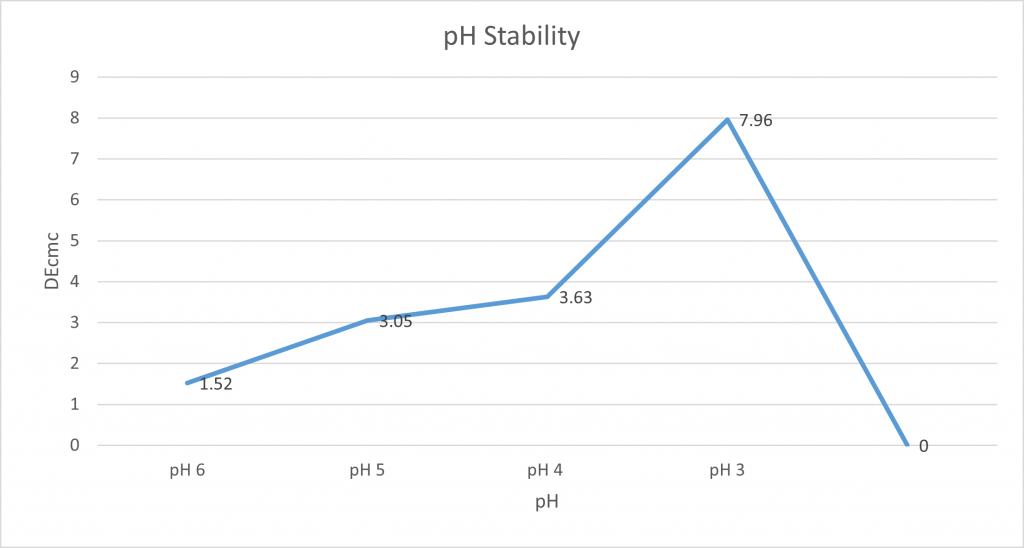
The results of our pH stability trial demonstrate that spirulina is the most stable at around neutral.
It is important to mention the link between temperature and pH – if spirulina is exposed to both these factors simultaneously it will affect the pigment twice as quickly. As spirulina has limited heat and acid stability, it must be used carefully and processed carefully to avoid damaging the pigment.
Suggested applications: Confectionery, Sports Nutrition, Bakery (icing and frostings), Dairy, Beverage.
In summary:
| pH | Limited |
| Heat | Limited |
| Light | Good |
If you have any questions about the research study our Colours Lab ran to test the stability of spirulina, or wish to request a sample of our spirulina, email us: sales@plant-ex.com
